All published articles of this journal are available on ScienceDirect.
The Impact Of Azo Dye Exposure On Nrf2 Activation And Oxidative Stress Markers In Textile Workers
Abstract
Background
Azo dyes are widely used in the textile industry for fabric coloring; however, their potential to induce oxidative stress poses significant health concerns. Nrf2 (Nuclear factor erythroid 2-related factor 2) is a pivotal oxidative stress marker.
Methods
This study involved 90 male workers: 45 exposed to azo dyes and 45 unexposed (control group). The exposed group was subdivided based on exposure duration into three categories: EG1 (1–10 years), EG2 (11–20 years), and EG3 (>20 years). Nrf2 activity was measured using ELISA, while Total Antioxidant Capacity (TAC) and Total Oxidative Status (TOS) were assessed via spectrophotometry.
Results
Exposed workers exhibited significantly higher Nrf2 activity (mean ± SD: 2.06 ± 0.68) than the control group (1.58 ± 0.41, P = 0.000). Additionally, the exposed group showed lower TAC (1.39 ± 0.1 vs. 1.50 ± 0.06, P = 0.000) and higher TOS (0.14 ± 0.05 vs. 0.11 ± 0.03, P = 0.002). Nrf2 levels increased with the duration of exposure (EG1: 1.68 ± 0.37, EG2: 1.92 ± 0.44, EG3: 2.60 ± 0.81), while TOS levels rose (EG1: 0.10 ± 0.04, EG2: 0.13 ± 0.03, EG3: 0.19 ± 0.02), and TAC levels decreased (EG1: 1.49 ± 0.06, EG2: 1.38 ± 0.05, EG3: 1.30 ± 0.09).
Discussion
The elevation of Nrf2, along with increased TOS and reduced TAC levels, suggests a compensatory antioxidant response to ongoing oxidative burden. These changes reflect the biological impact of chronic exposure to azo dyes.
Conclusion
These findings highlight the role of azo dyes in inducing oxidative stress among textile workers, emphasizing the urgent need for protective measures to mitigate occupational health risks.
1. INTRODUCTION
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are crucial for aerobic organisms, produced as byproducts of metabolic processes. ROS, including superoxide anion (O2•−), peroxy radical (ROO•), hydroxyl radical (HO•), hydrogen peroxide (H2O2), and singlet oxygen (1O2), are key players in cellular signaling and homeostasis [1]. Similarly, RNS, such as nitric oxide (NO), nitroxyl (HNO), nitrogen dioxide (NO2), and peroxynitrite (ONOO–), are involved in regulatory processes but can lead to nitrosative stress when present in excess [2].
The balance between ROS/RNS production and the body's antioxidant defense systems—such as catalase, superoxide dismutase (SOD), and glutathione—is vital for maintaining redox homeostasis. Under normal physiological conditions, antioxidants neutralize harmful ROS/RNS; however, when excessive production occurs during pathological states, it leads to oxidative stress, as shown in Fig. (1), which disrupts redox balance and contributes to various diseases [3, 4].
1.1. Nuclear Factor Erythroid 2-related Factor 2 (Nrf2)
Nrf2 is a key transcription factor that regulates the cellular response to oxidative stress by binding to the antioxidant response element (ARE) in the promoter regions of genes encoding antioxidant proteins and detoxification enzymes [6]. Nrf2 controls the production of antioxidants and protects the body from oxidative stress and inflammation. Under normal conditions, Nrf2 forms a complex with Keap1 in the cytoplasm, which prevents its entry into the nucleus and promotes degradation. Keap1, a Kelch-like ECH-associated protein 1, serves as a substrate adaptor protein [7, 8]. In healthy cells, Nrf2 levels remain low, and its target genes are expressed at baseline levels [9]. However, under oxidative stress, Nrf2 dissociates from Keap1, translocates to the nucleus, and activates a range of target genes that help mitigate oxidative damage [10], as shown in Fig. (2).
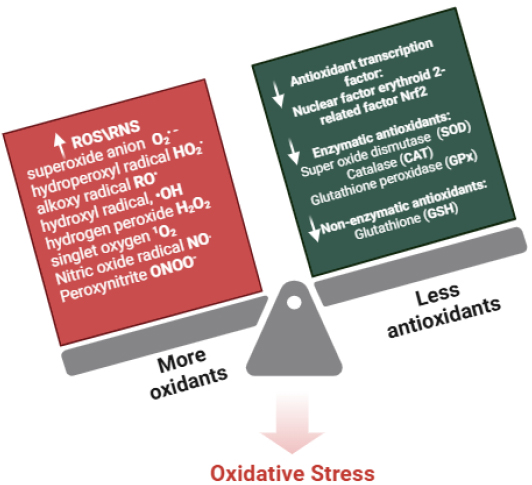
Factors influencing redox imbalance and key oxidative stress markers, highlighting the relationship between reactive oxygen species (ROS), reactive nitrogen species (RNS), and antioxidant defenses in cellular homeostasis. Adapted and modified from [5].
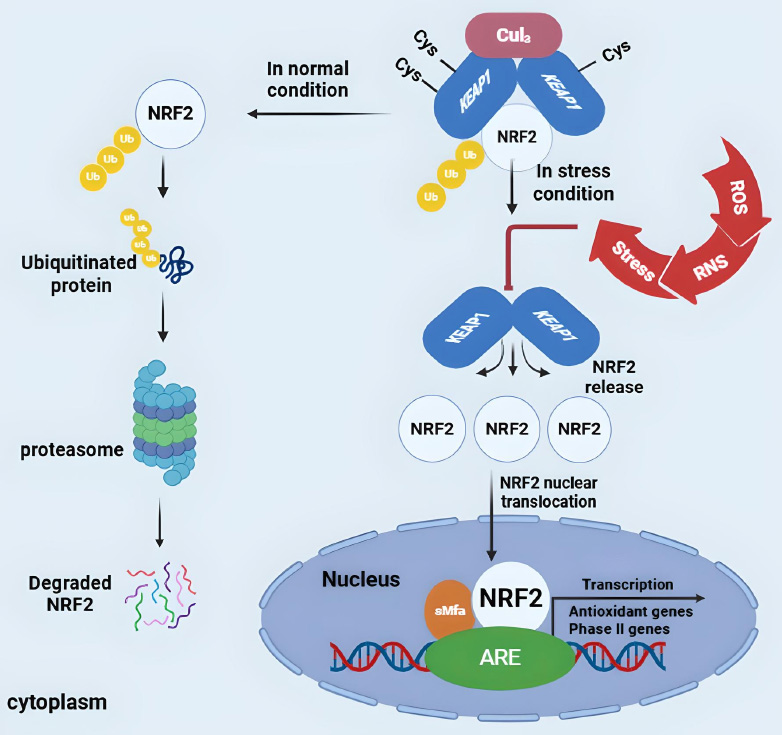
The Keap1-Nrf2-ARE signaling pathway. Under basal conditions, Keap1 inhibits Nrf2 by promoting its degradation. Upon oxidative stress, Nrf2 dissociates from Keap1, translocates to the nucleus, and activates antioxidant genes (e.g., SOD, CAT, GSH, HO-1) to counteract oxidative damage. Adapted and modified from [11].
1.2. Azo Dyes
Azo dyes are aromatic compounds characterized by one or more azo bonds (-N=N-) within their molecular structure [12]. These dyes are commonly used in various industries, including textiles, pharmaceuticals, food, and cosmetics, due to their cost-effectiveness, ease of production, and wide range of colors [13, 14]. Textile dyes are categorized into two types: natural and synthetic, as shown in Fig. (3) [15]. In the textile industry, synthetic azo dyes are predominantly used to color fabrics, contributing to environmental pollution through effluent discharge. This poses significant risks to aquatic ecosystems and human health [16, 17]. Moreover, azo dyes have the potential to generate carcinogenic aromatic amines when degraded, which further exacerbates their harmful effects on health [18]. The environmental and health risks associated with azo dyes have prompted research into bioremediation strategies to break down these harmful compounds, with enzymes such as azoreductases receiving increasing attention for their role in detoxifying azo dyes [19].
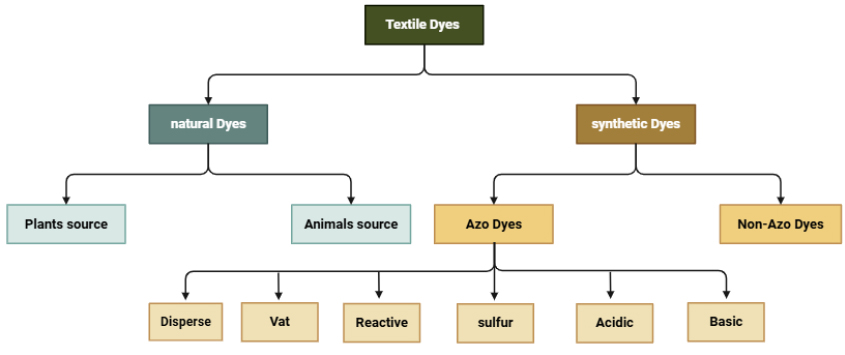
Classification of textile dyes used in the textile industry. Adapted and modified from [15].
In the literature review, textile dyeing may lead to respiratory symptoms in workers [20]. Workers are exposed to health risks related to ear, nose, and throat diseases, skin infections, and eye symptoms due to their daily exposure to hazardous chemicals [21]. MB-5G and RB-203 textile dyes in wastewater released into the ecosystem cause genotoxic and teratogenic effects in the developmental stages of zebrafish larvae [22]. E. coli could be suitable for treating textile wastewater containing azo dyes [23]. UV light, along with hydrogen peroxide and TiO2, creates the best conditions to break down Allura Red AC dye [24]. Possible effects of occupational exposure to vat-textile dyes on reproductive hormones in females in the follicular and luteal stages appear to be associated with age and duration of exposure [25].
Here, Despite the widespread use of azo dyes in the textile industry, the specific health risks associated with their exposure, particularly concerning oxidative stress, remain underexplored. This study aims to address this gap by investigating the effects of azo dyes on textile workers, with a focus on how these dyes influence Nrf2 activation and oxidative stress markers. By understanding the molecular mechanisms through which azo dyes impact oxidative stress and Nrf2 function, this research seeks to inform strategies for improving occupational health and safety standards in the textile industry.
2. MATERIALS
3. METHODS
3.1. Blood Sample Collection
Disposable syringes and needles were employed for blood collection. A total of 5 mL of whole blood samples were obtained from both exposed and unexposed (control) groups and placed in gel tubes. The blood samples were allowed to clot before being centrifuged at 500 rpm for 5 minutes. The serum was then separated, divided into fractions in Eppendorf tubes, and stored at -20°C until analysis.
3.2. Study Design
An evaluative study was conducted involving 90 male textile workers at Hilla Textile Industry. The participants were divided into 45 exposed workers and 45 unexposed (control) workers to azo dyes. The exposed workers were further categorized based on the duration of exposure into three subgroups, as illustrated in Fig. (4):
- EG1: 1-10 years (age 38.3±13.8).
- EG2: 11-20 years (age 46.4±8.2).
- EG3: more than 20 years (age 54.1±5.3).
The study considered several criteria, including age, duration of work, social status, number of children, medical history, and whether the workers' environment was rural or urban.
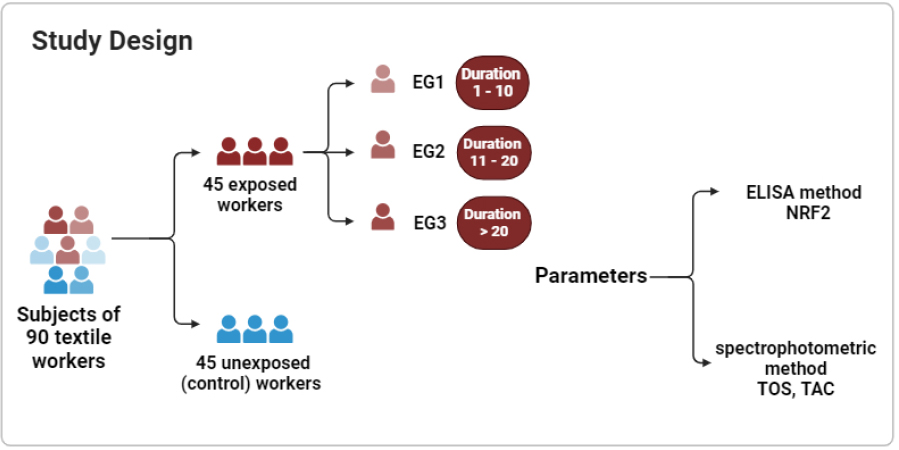
Study design, showing participants grouped based on Exposure duration to Azo Dyes divided into exposed and control groups. The exposed group was further stratified into subgroups based on exposure duration: EG1 (1–10 years), EG2 (11–20 years), and EG3 (>20 years).
The sample size for this study (N = 90) was chosen to create a balanced distribution between the exposed and control groups, allowing for robust statistical comparisons. By selecting 45 participants in the exposed group and 45 in the control group, we can adequately represent various exposure durations while keeping data collection and biomarker analysis manageable. This balanced distribution enhances the reliability of the findings by minimizing bias and ensuring that variations in oxidative stress markers can be effectively assessed across the different groups and Nrf2 activation, allowing for a thorough evaluation of the effects of azo dye exposure on redox homeostasis.
3.3. Assay of NRF2 (Elisa Assay)
All reagents, samples, and standards were prepared according to the instructions provided in the ELISA kit (human Thymus activation regulated chemokine (TARC) manual from Biont. Samples, standards, and ELISA solutions were added and allowed to react for 60 minutes at 37°C. The plate was washed five times. Chromogen solutions A and B were added, and the mixture was incubated for 10 minutes at 37°C to develop color. A stop solution was added. The absorbance value was read at 450 nm using a microplate reader within 10 minutes of adding the stop solution. As shown in the work steps in Fig. (5), and preparation of standard solutions shown in Fig. (6).
3.3.1. Calculation
The dose-response standard curve is utilized to assess NRF2 concentration in serum, as shown in Fig. (7).
3.4. TAC and TOS Assays (Spectrophotometric Assay)
TAC and TOS levels were quantified using specific methods. The total antioxidant capacity (TAC) was determined using the CUPRAC method [26], while total oxidative status (TOS) was assessed using the Erel method (2005) [27]. Detailed procedures for TAC and TOS assays, including the reagents used and their respective quantities, are presented in Tables 1 and 2.
| Reagents | Test | STD | Blank |
|---|---|---|---|
| Copper (II) chloride solution | 1000 µL | 1000 µL | 1000 µL |
| Serum | 50µL | ----- | ----- |
| Uric acid Solution | ----- | 50µL | ----- |
| D.W | ----- | ----- | 50µL |
| Neocuproine (Nc) solution | 1000 µL | 1000 µL | 1000 µL |
| Ammonium acetate (NH4Ac) buffer | 1000 µL | 1000 µL | 1000 µL |
| Test tubes were mixed by vortex and incubated for 30 minutes at 37 °C, then centrifuged at 4000 rpm for 2 minutes; after that, the absorbance was measured using a spectrophotometer at 450 nm. | |||
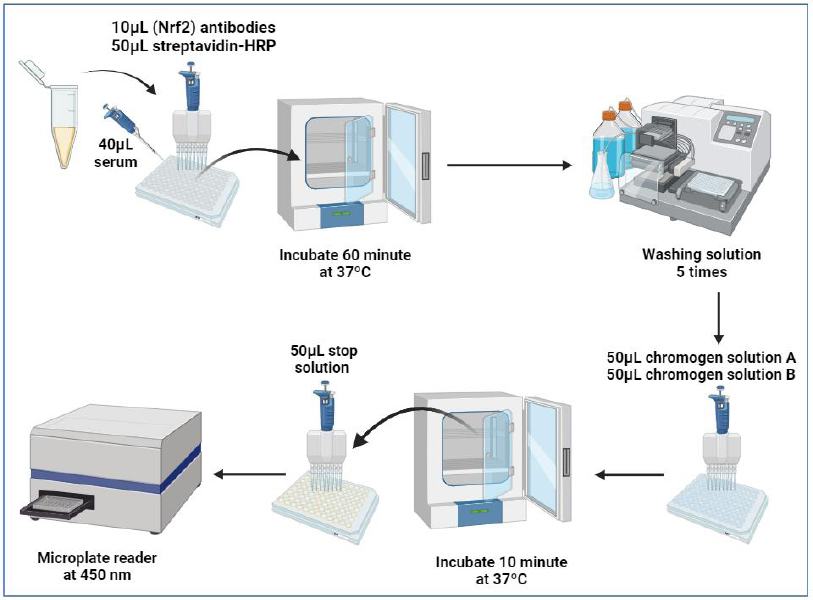
Schematic of the ELISA procedure for NRF2 measurement, outlining sample preparation, incubation, washing, and absorbance measurement at 450 nm.
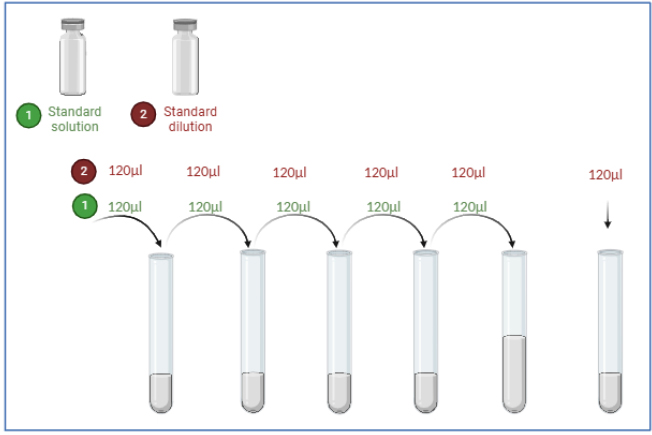
Diagram showing procedures for standard solution preparation and serial dilution required for NRF2 ELISA quantification.
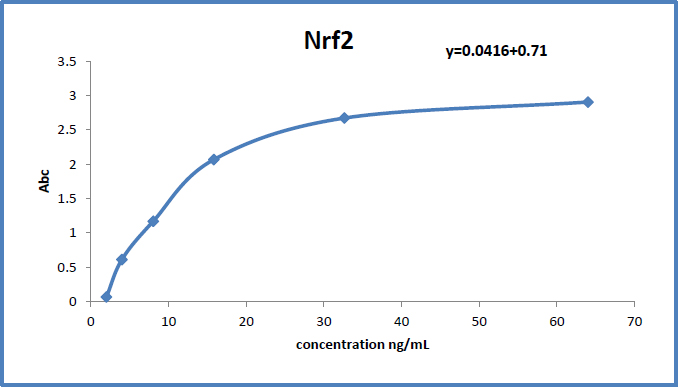
Standard curve showing the relationship between absorbance and NRF2 concentration in serum samples. The figure is used to determine NRF2 levels in the study participants.
| Reagents | Blank | STD | Test |
|---|---|---|---|
| D.W | 25 µL | ----- | ----- |
| Serum | ----- | ----- | 25 µL |
| H2O2 | ----- | 25µL | ----- |
| R1 | 1000 µL | 1000 µL | 1000 µL |
| Test tubes were mixed by vortex, then added | |||
| R2 | 250 µL | 250 µL | 250 µL |
3.5. Statistical Analysis
Statistical analyses were conducted using SPSS (Statistical Package for the Social Sciences) version 25 and GraphPad Prism version 8.0.2 software. The Kolmogorov-Smirnov test was utilized to assess whether the data followed a normal distribution. Data are presented as means ± standard deviation (SD). One-way analysis of variance (ANOVA) with Tukey’s post hoc test was employed to compare continuous variables. A Student’s t-test was also used for statistical comparisons between experimental groups. A p-value of less than 0.05 (p < 0.05) was considered statistically significant.
4. RESULTS
4.1. Characteristics of the Exposed Group and Control Group
Table 3 presents the baseline characteristics of the study participants, including data for both the exposed group and the control group. The table includes arithmetic results for measurements of Nuclear Factor Erythroid 2-related Factor 2 (NRF2), Total Antioxidant Capacity (TAC), and Total Oxidant Status (TOS). The study comprised 90 workers, evenly divided with 45 in the exposed group (age 46.04±11.39) and 45 in the control group (age 51.2±7.3).
| Variables | Groups | NO. | Mean ± SD | 95% Confidence Interval | P-value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| NRF2 ng/mL | Exposed | 45 | 2.06±0.68 | 1.86 | 2.27 | 0.000 |
| Control | 45 | 1.58±0.41 | 1.46 | 1.70 | ||
| TAC mM | Exposed | 45 | 1.39±0.10 | 1.36 | 1.42 | 0.000 |
| Control | 45 | 1.50±0.06 | 1.48 | 1.51 | ||
| TOS mM | Exposed | 45 | 0.14±0.05 | 0.12 | 0.15 | 0.002 |
| Control | 45 | 0.11±0.03 | 0.10 | 0.12 | ||
The differences between the exposed and control groups were statistically significant (P<0.05) for all parameters: NRF2 (P=0.000), TAC (P=0.000), and TOS (P=0.002).
4.1.1. Interpretation of Results
4.1.1.1. -NRF2 (Nuclear Factor Erythroid 2-related Factor 2)
• Exposed Group: 2.06 ± 0.68 ng/mL
• Control Group: 1.58 ± 0.41 ng/mL
The results show a significant increase in NRF2 levels in the exposed group compared to the control group (P=0.000). This elevation likely indicates an adaptive response to oxidative stress caused by environmental exposure or occupational exposure.
In 2021, Leyi Ma et al. concluded that baicalin (BAI), a natural flavonoid, may be effective in treating diabetic nephropathy (DN). Their findings suggested that BAI alleviates oxidative stress and inflammation through two main mechanisms: activating the Nrf2-mediated antioxidant signaling pathway and inhibiting the MAPK (mitogen-activated protein kinase)-mediated inflammatory signaling pathway [28]. In 2021, Xianlei Jiang et al. conducted a study on the effects of lead exposure on the female reproductive system, focusing specifically on oocyte maturation and fertility in mice. They also examined the role of Nrf2-mediated defense responses during lead exposure. The researchers discovered that lead exposure leads to oxidative stress, characterized by decreased glutathione levels and increased reactive oxygen species. To protect oocytes from this oxidative stress, the body activates the Nrf2 signaling pathway, which enhances the transcription of antioxidant enzymes [29].
4.1.1.2. Oxidative Stress
TAC (Total Antioxidant Capacity):
• Exposed Group: 1.39 ± 0.10 mM
• Control Group: 1.50 ± 0.06 mM
The exposed group showed a decrease in TAC (P=0.000), indicating a reduced ability to counteract oxidative stress, likely due to exposure to oxidative agents in the environment.
4.1.1.3. TOS (Total Oxidant Status):
• Exposed Group: 0.14 ± 0.05 mM
• Control Group: 0.11 ± 0.03 mM
The significantly higher TOS levels in the exposed group (P=0.002) support the hypothesis of increased oxidative stress in this group, which may lead to long-term cellular damage.
In 2020, Pritee Chaudhary et al. indicated that biomarkers of oxidative stress (OS) play a significant role in the pathophysiology of hypertension (HTN) and that endothelial dysfunction is also linked to this condition. They evaluated oxidative stress by measuring total oxidative status (TOS) and total antioxidant capacity (TAC), while endothelial dysfunction was assessed through flow-mediated dilation (FMD) [30]. The researchers found that TOS levels were significantly elevated, while TAC and FMD levels were significantly reduced in hypertensive patients, with these effects becoming more pronounced with age. They concluded that decreased TAC and increased TOS indicate oxidative stress, which may contribute to the diminished FMD observed in older hypertensive patients [30]. In 2024, Amal Saad-Hussien et al. evaluated the impact of endogenous antioxidants on oxidative stress caused by occupational exposures in the textile dyeing industry and its effects on the liver of exposed workers. They discovered a significant association between the duration of exposure and the workers' age. The researchers concluded that occupational exposure to chemicals during dyeing processes may harm the liver of exposed workers through oxidative stress [31].
4.2. Biochemical Parameter
4.2.1. Influence of Exposure Duration on Biochemical Markers
This study examines the impact of exposure duration on oxidative stress biomarkers, using ANOVA to assess significant differences among the studied groups. Participants were categorized based on exposure duration as outlined in the study design.
In Table 4, the results demonstrate a significant increase in Nuclear Factor Erythroid 2-Related Factor 2 (NRF2) levels with prolonged exposure to azo dyes. NRF2 was highest in Group 3 (EG3: >20 years, 2.60 ± 0.81 ng/mL) and lowest in Group 1 (EG1: 1-10 years, 1.68 ± 0.37 ng/mL). This trend suggests that prolonged exposure may enhance NRF2 activation, potentially indicating an adaptive cellular response to oxidative stress.
Similarly, Total Antioxidant Capacity (TAC) levels exhibited a decreasing trend with increasing exposure duration. Workers in EG1 had the highest TAC levels (1.49 ± 0.06 mM), whereas those in EG3 had the lowest (1.31 ± 0.09 mM). This decline suggests that long-term exposure to azo dyes may impair the antioxidant defense system, leading to increased oxidative stress vulnerability.
| Variables | Groups | NO. | Mean ± SD | 95% Confidence Interval | P-value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| NRF2 | EG1 (1-10) | 15 | 1.68 ± 0.37 | 1.47 | 1.88 | 0.0001 |
| EG2 (11-20) | 15 | 1.92 ± 0.44 | 1.67 | 2.16 | ||
| EG3 (>20) | 15 | 2.60 ± 0.81 | 2.15 | 3.05 | ||
| TAC | EG1 (1-10) | 15 | 1.49±0.06 | 1.45 | 1.52 | 0.0001 |
| EG2 (11-20) | 15 | 1.38±0.05 | 1.35 | 1.41 | ||
| EG3 (>20) | 15 | 1.31±0.09 | 1.25 | 1.36 | ||
| TOS | EG1 (1-10) | 15 | 0.10±0.04 | 0.08 | 0.13 | 0.002 |
| EG2 (11-20) | 15 | 0.13±0.03 | 0.11 | 0.14 | ||
| EG3 (>20) | 15 | 0.19±0.02 | 0.17 | 0.20 | ||
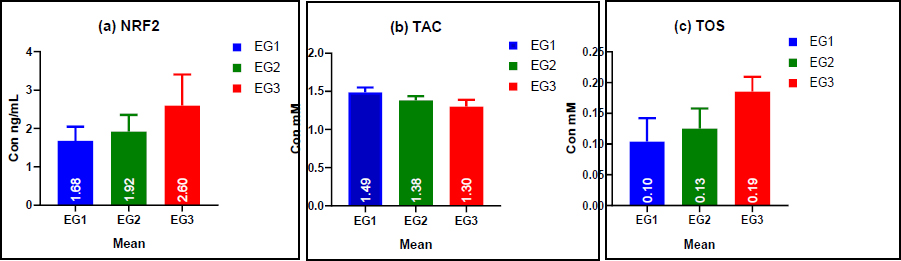
Bar graphs illustrating the mean levels of (a) NRF2, (b) TAC, and (c) TOS in the study groups. The figure indicates significant differences in oxidative stress biomarkers between the exposed and control groups. As the duration of exposure increases, NRF2 and TOS levels rise, while TAC levels decrease.
Conversely, Total Oxidant Status (TOS) levels progressively increased with longer exposure duration. The highest levels were observed in EG3 (0.19 ± 0.02 mM), significantly higher than in EG1 (0.10 ± 0.04 mM) and EG2 (0.13 ± 0.03 mM). These findings support the hypothesis that prolonged exposure to azo dyes disrupts redox homeostasis, leading to increased oxidative stress and potential cellular damage Fig. (8).
4.2.2. Multiple Comparisons of NRF2, TAC, and TOS Levels among the Studied Groups
Tukey's post hoc test was used, as presented in Table 5, to further assess the significance of the differences in levels of NRF2, TAC, and TOS among the various exposure groups. The results offer a clearer understanding of how oxidative stress biomarkers are influenced by different durations of exposure to azo dyes.
| Variables | Groups | Compared Groups | Mean | P-value | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|---|
| Difference | Lower | Upper | |||||
| NRF2 | EG1 (1-10) | EG1 | EG2 | -0.24 | 0.49 | -0.75 | 0.27 |
| EG2 (11-20) | EG3 | -0.92* | 0.0002 | -1.43 | -0.41 | ||
| EG3 (>20) | EG2 | EG3 | -0.68* | 0.006 | -1.19 | -0.17 | |
| TAC | EG1 (1-10) | EG1 | EG2 | 0.11* | 0.001 | 0.04 | 0.17 |
| EG2 (11-20) | EG3 | 0.18* | 0.00001 | 0.12 | 0.24 | ||
| EG3 (>20) | EG2 | EG3 | 0.08* | 0.01 | 0.01 | 0.14 | |
| TOS | EG1 (1-10) | EG1 | EG2 | -0.02 | 0.17 | -0.05 | 0.01 |
| EG2 (11-20) | EG3 | -0.08* | 0.00001 | -0.11 | -0.05 | ||
| EG3 (>20) | EG2 | EG3 | -0.06* | 0.00002 | -0.09 | -0.03 | |
The levels of NRF2 showed a significant increase with longer exposure durations. Although there was no notable difference between workers exposed for 1 to 10 years (EG1) and those exposed for 11 to 20 years (EG2) (P = 0.49), a significant rise in NRF2 levels was observed in individuals exposed for more than 20 years (EG3) compared to both EG1 (P = 0.0002) and EG2 (P = 0.006). This indicates that NRF2 activation intensifies only after prolonged exposure, likely serving as an adaptive cellular response to accumulated oxidative stress. The delayed upregulation of NRF2 suggests that the antioxidant defense system is continuously challenged over time, requiring enhanced protective mechanisms.
In contrast, Total Antioxidant Capacity (TAC) levels showed a progressive decline with longer exposure durations. A significant decrease in TAC was noted between EG1 and EG2 (P = 0.001), indicating that antioxidant depletion begins within the first decade of exposure. This reduction became even more pronounced in EG3 than EG2 (P = 0.00001), further supporting the idea that long-term exposure diminishes the antioxidant defense system. Additionally, the difference between EG1 and EG3 (P = 0.01) confirms that antioxidant reserves continue to decline cumulatively with prolonged exposure, making workers increasingly vulnerable to oxidative stress.
A significant increase in Total Oxidative Status (TOS) was observed in workers with >20 years of exposure. Specifically, when comparing EG2 vs. EG3, TOS increased significantly (P = 0.00001), indicating higher oxidative stress levels associated with longer exposure durations. In contrast, no significant difference was found between EG1 and EG2 (P = 0.17), suggesting that oxidant levels remain relatively stable during the first decade of exposure. However, when comparing EG1 to EG3, a significant increase in TOS was observed (P = 0.00002), indicating that chronic exposure to azo dyes significantly elevates oxidative stress levels.
These findings show that prolonged exposure to azo dyes disrupts redox homeostasis, shown by increased NRF2 levels as an adaptive response, decreased TAC indicating weakened antioxidant defenses, and increased TOS reflecting intensified oxidative stress. These changes underscore the cumulative nature of oxidative damage, highlighting the need for preventive strategies and protective measures for textile workers exposed to azo dyes. Mondal et al. (2018) found that biomass fuel (BMF) users exhibited increased reactive oxygen species (ROS) in blood neutrophils and sputum cells, coupled with decreased erythrocyte superoxide dismutase (SOD) and relatively unchanged plasma catalase, indicating oxidative stress. In these users, Keap1 expression was reduced, and nuclear Nrf2 expression increased two- to three-fold. These findings suggest that BMF cooking activates Nrf2, potentially as an adaptive cellular response to oxidative stress and inflammation-related airway injury caused by biomass smoke [32].
4.3. Pearson correlation between NRF2, TAC, and TOS
The Pearson correlation analysis was conducted to evaluate the relationships among NRF2, TAC, and TOS, as shown in Table 6. These correlations provide insights into the oxidative stress response and antioxidant defense mechanisms in textile workers exposed to azo dyes.
| Variables | NRF2 | |
|---|---|---|
| R | P-value | |
| TAC | -0.578** | 0.00003 |
| TOS | 0.539** | 0.0001 |
The results revealed a significant negative correlation between NRF2 and total antioxidant capacity (TAC) (r = -0.578, P = 0.00003). This indicates that as NRF2 levels increase, TAC levels decrease. Despite the activation of NRF2 in response to oxidative stress, the overall antioxidant capacity appears to diminish. This decline may result from a prolonged oxidative burden that exceeds the body's ability to replenish its antioxidants. Consequently, TAC decreases over time, suggesting that the antioxidant defense system is overwhelmed and unable to effectively counteract reactive oxygen species (ROS).
Additionally, a significant positive correlation was observed between NRF2 and TOS (r = 0.539, P = 0.0001), indicating that higher levels of NRF2 are associated with increased oxidative stress (TOS). This suggests that NRF2 is upregulated as a compensatory response to heightened oxidative stress. However, the ongoing increase in TOS, even with NRF2 activation, underscores the severity of oxidative damage, as the antioxidant defense mechanisms may not be able to fully counteract the excessive production of reactive oxygen species (ROS).
These findings highlight NRF2's role in oxidative stress adaptation and indicate that prolonged exposure to azo dyes disrupts redox balance, lowering TAC levels and leading to persistent oxidative stress. Continuous NRF2 activation suggests an ongoing attempt to combat oxidative damage, but depleting TAC indicates exhausted antioxidant reserves. Zhang et al. (2021) found a positive correlation between apoptosis, the Nrf2 pathway, and oxidative stress, suggesting that T-2 toxin-induced nephrotoxicity in mice, mediated by oxidative stress-induced apoptosis, is related to the Nrf2 pathway [33].
The strong correlations underline the importance of monitoring oxidative stress markers in occupational studies and the need for interventions to enhance antioxidant defenses, such as dietary changes, supplementation, or better workplace protections, to reduce chronic oxidative stress effects in workers.
5. DISCUSSION
This study highlights the significant impact of azo dyes on oxidative stress levels among textile workers, evidenced by the cellular response of Nrf2. Our results show that workers exposed to azo dyes exhibit increased NRF2 activity compared to the control group. Nrf2 is essential for cellular defense against oxidative stress, regulating antioxidant enzymes and detoxification proteins. The increased NRF2 activity in exposed workers indicates an adaptive response to mitigate the oxidative burden from azo dyes. Doumet Georges Helou et al. (2019) highlighted Nrf2's protective role in mitigating oxidative stress and inflammation, emphasizing its crucial regulation of the skin's innate immune response to chemical sensitizers [34].
Our study revealed differences in NRF2 activity along with contrasting levels of Total Antioxidant Capacity (TAC) and Total Oxidant Status (TOS) between the exposed and control groups. The exposed group exhibited significantly lower TAC and higher TOS levels, indicating reduced antioxidant defenses and increased oxidative damage due to azo dye exposure. These findings highlight the need for effective occupational health measures to mitigate oxidative stress and health risks for textile workers. Ying et al. (2024) found that metal fume ultrafine particulates (MFUFPs) induce oxidative stress and activate the Nrf-2/ARE pathway to combat oxidative damage [35].
Furthermore, the different responses seen in NRF2, TAC, and TOS levels highlight the intricate nature of oxidative stress pathways affected by occupational exposure to azo dyes. Future research needs to delve into the molecular mechanisms that stimulate Nrf2 and the oxidative stress pathways in textile environments to devise specific interventions and protective strategies.
Adiponectin, a hormone secreted by adipose tissue and known for its anti-inflammatory and antioxidant properties, plays a vital role in reducing oxidative stress. It boosts antioxidant defenses and suppresses pro-inflammatory cytokines [36, 37]. The observed decline in TAC and increase in TOS in exposed workers may suggest a changed response to adiponectin. Although this study did not measure adiponectin levels, future research should consider it as a potential biomarker to gain a better understanding of the metabolic and inflammatory effects of azo dye exposure. Assessing adiponectin levels alongside oxidative stress markers (Nrf2, TAC, TOS) and inflammatory mediators (CCL17, cytokines) may yield deeper insights into the systemic effects of occupational exposure to textile dyes.
6. LIMITATIONS OF THE STUDY
This study primarily focused on exposure duration rather than chronological age as a determining factor in variations of oxidative stress. While the number of years of exposure provides a solid basis for assessing occupational risk, age-related physiological changes could also influence antioxidant levels and oxidative stress responses. Future research should incorporate age as an additional variable to better understand its role in modulating oxidative stress.
Additionally, while Total Antioxidant Capacity (TAC) and Total Oxidative Status (TOS) were used as key markers of oxidative stress, the study did not include advanced antioxidant capacity assays such as the Total Radical-Trapping Antioxidant Parameter (TRAP) and the Oxygen Radical Absorbance Capacity (ORAC). Incorporating these assays in future studies would offer a more comprehensive evaluation of the oxidative balance.
Furthermore, the study concentrated on oxidative stress biomarkers (Nrf2, TAC, and TOS) without evaluating inflammatory markers such as adiponectin, cytokines, and chemokines. These markers could provide deeper insights into the metabolic and immune responses related to azo dye exposure. Lifestyle factors, including diet, smoking, and Body Mass Index (BMI), which may influence oxidative stress levels, were not considered in this research. Additionally, while exposure duration was a key variable, other occupational and environmental co-exposures were not assessed. Future research should integrate a broader range of biomarkers and environmental factors to offer a more comprehensive understanding of the health risks associated with azo dye exposure.
7. RECOMMENDATIONS
To enhance our understanding of the health risks associated with exposure to azo dyes, future studies should focus on the following areas:
• Evaluating Additional Biomarkers: Incorporate more oxidative stress biomarkers, such as Total Radical-Trapping Antioxidant Parameter (TRAP) and Oxygen Radical Absorbance Capacity (ORAC), to obtain a comprehensive assessment of oxidative balance.
• Investigating Inflammatory Biomarkers: Explore the roles of adiponectin and other inflammatory biomarkers (such as cytokines and chemokines) to better understand the relationship between oxidative stress and inflammation in workers exposed to azo dyes.
• Examining Lifestyle and Individual Factors: Assess the impact of various lifestyle and individual factors—like body mass index (BMI), socioeconomic status, number of children, medical history, and living environment—on oxidative stress levels (Total Antioxidant Capacity and Total Oxidant Status) to identify potential risk modifiers.
• Conducting Clinical Assessments: Perform clinical evaluations to determine the long-term health effects of azo dye exposure, particularly concerning respiratory, gastrointestinal, and neurological symptoms, in textile workers with prolonged exposure.
CONCLUSION
The study demonstrates that prolonged exposure to azo dyes in the textile industry leads to significant changes in oxidative stress markers. The increased NRF2 levels suggest an adaptive response to combat oxidative stress, while the reduced TAC and elevated TOS levels reflect an overwhelmed antioxidant defense system and increased oxidative damage. The results underscore the need for effective occupational health measures to mitigate the harmful effects of azo dye exposure.
By understanding the molecular mechanisms, particularly the role of NRF2 in oxidative stress, future interventions can be developed to protect workers in environments with high exposure to hazardous chemicals like azo dyes.
DECLARATION OF GENERATIVE AI AND AI-ASSISTED TECHNOLOGIES IN THE WRITING
The English language of the article was improved with ChatGPT.
The author generated this text in part with GPT-3, OpenAI’s large-scale language-generation model. Upon generating draft language, the author reviewed, edited, and revised the language to their own liking and takes ultimate responsibility for the content of this publication.
During the preparation of this work the author(s) used GPT-3/ OpenAI in order to enhance the writing and language.
LIST OF ABBREVIATIONS
| ROS | = Reactive oxygen species |
| RNS | = Reactive nitrogen species |
| SOD | = Superoxide dismutase |
| ARE | = Antioxidant response element |
| TAC | = Total Antioxidant Capacity |
| TOS | = Total Oxidative Status |
| SD | = Standard deviation |
| NRF 2 | = Nuclear Factor Erythroid 2-related Factor 2 |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study is approved by the Scientific Committee, Department of Chemistry, College of Science, University of Babylon, Iraq (Approval Number/Date: 1125 (09/07/2024)).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of this article are available upon request from the corresponding author [Y.K.S].
ACKNOWLEDGEMENTS
Y.K.S. expresses gratitude to the University of Babylon in Iraq for its support while pursuing the MSc degree. Additionally, thanks are extended to Professor Lamia Abdul-Majeed Al-Mashhady from the Chemistry Department for providing the opportunity to complete this project and for her invaluable support. Special thanks are also extended to Ayad Ali Disher for his guidance and valuable contributions throughout the study.


